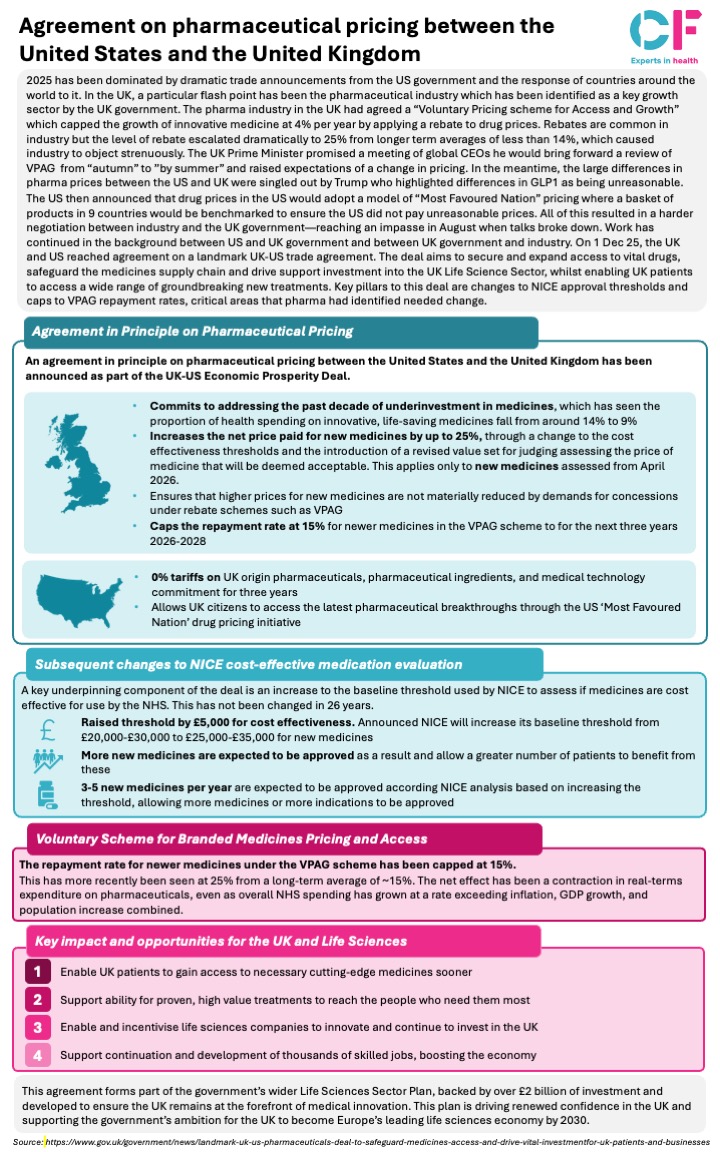
The UK and US governments have announced a landmark agreement in principle on pharmaceutical pricing as part of the UK-US Economic Prosperity Deal, following a year of intense negotiations. The pharmaceutical industry had been operating under the “Voluntary Pricing scheme for Access and Growth” (VPAG) which saw rebate levels escalate dramatically to 25% from long-term averages of less than 14%, causing industry to object strenuously. Meanwhile, large differences in pharma prices between the US and UK were singled out by Trump, leading to the US announcement of “Most Favoured Nation” pricing. After negotiations reached an impasse in August when talks broke down, work continued in the background between governments and industry.
On 1 December 2025, the agreement was reached, aiming to secure and expand access to vital drugs for tens of thousands of NHS patients, safeguard the medicines supply chain, and drive crucial investment into the UK Life Science Sector, whilst enabling UK patients to access a wide range of groundbreaking new treatments.
Key pillars to this deal are changes to NICE appraisal thresholds and caps to VPAG repayment rates, which come 9 months after the UK government promising a VPAG review by ‘summer’. The agreement forms part of the government’s wider Life Sciences Sector Plan, backed by over £2 billion of investment and developed to ensure the UK remains at the forefront of medical innovation, supporting the government’s ambition for the UK to become Europe’s leading life sciences economy by 2030.
The agreement addresses the past decade of underinvestment in medicines, which has seen the proportion of health spending on innovative, life-saving medicines fall from around 14% to 9%.
Key elements include:
Changes to NICE cost-effective dedication evaluation
NICE’s baseline threshold—unchanged for 26 years—will increase from £20,000-£30,000 to £25,000-£35,000 for new medicines. This is expected to enable recommendation of an additional 3-5 new medicines or indications per annum, allowing more patients to benefit from new treatments.
Key impact and opportunities
The agreement is designed to deliver four key benefits for the UK and Life Sciences sector:
- Enable UK patients to gain access to necessary cutting-edge medicines sooner
- Support ability for proven, high value treatments to reach the people who need them most
- Enable and incentivise life sciences companies to innovate and continue to invest in the UK
- Support continuation and development of thousands of skilled jobs, boosting the economy
Download our snapshot below for a full summary:
What this means for patients
For NHS patients, this agreement promises improved access to innovative, life-saving medicines that may previously have been deemed not cost-effective under the old NICE thresholds. The increase in baseline thresholds could enable 3-5 additional new medicines or indications to be approved each year, potentially benefiting tens of thousands of patients who would otherwise have limited treatment options.
However, the agreement raises questions about sustainability. The increase in net prices for new medicines by up to 25%, combined with raised NICE thresholds, will place additional pressure on the NHS medicines budget at a time when the health service is already under considerable financial strain. This raises concerns about whether the additional costs can be absorbed without affecting patient care in other areas, and whether the balance struck between patient access, pharmaceutical industry incentives, and NHS affordability is sustainable in the long term.
Read the full government announcement here.