Study on the deployment of AI in healthcare across the EU
Artificial Intelligence (AI) is rapidly transforming healthcare across Europe, promising to revolutionise diagnostics, patient care, and operational efficiency. The European Commission’s recent study, conducted by PwC EU and Open Evidence, provides a sweeping analysis of AI’s current state, future potential, and the challenges that must be overcome for Europe to lead in global health innovation. CF has expanded its snapshot service to cover European markets, providing a concise summary of the 241-page final report key findings and implications.
Why did the European Commission act?
Europe faces mounting pressures:
- Ageing populations and rising demand for healthcare services
- Resource constraints and workforce shortages
- Global competition from the US, UK, and China in health technology innovation
The EU’s motivation is clear: to harness AI as a strategic asset, ensuring safe, effective, ethical, and equitable deployment that supports system sustainability and future needs.
Key headlines
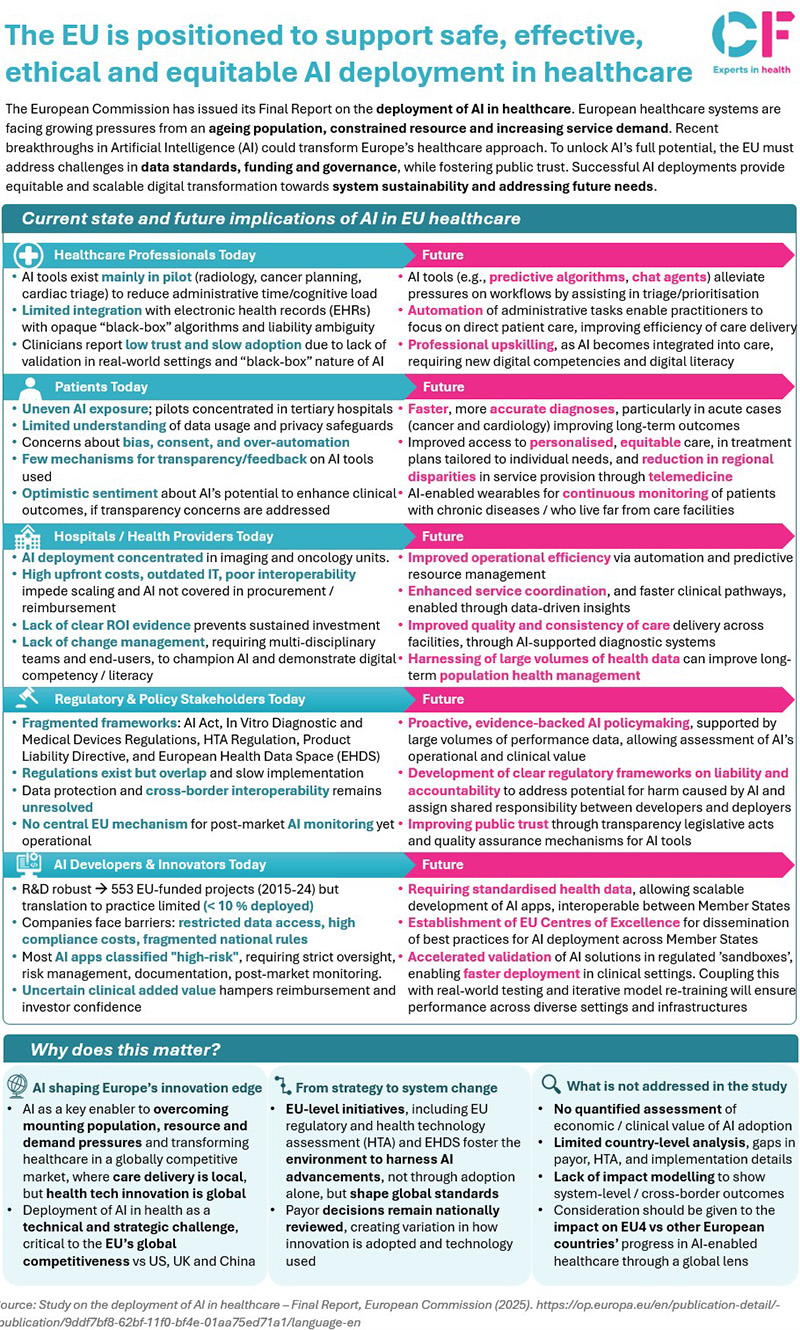
- AI’s transformative potential AI is already making waves in pilot projects—radiology, cancer planning, cardiac triage—reducing administrative burden and supporting clinical decision-making. Predictive algorithms and chat agents are streamlining workflows, enabling clinicians to focus on patient care.
- Barriers to adoption Despite promise, adoption is slow. Fragmented and outdated data systems, complex regulatory frameworks, limited funding, and public concerns over transparency and privacy are major hurdles. Most AI tools remain in pilot stages, with limited integration into electronic health records and real-world clinical settings.
- Stakeholder perspectives. The study identifies five key stakeholder groups, each with unique interests and challenges:
- Patients: AI offers earlier, more accurate diagnoses and personalised care, yet concerns about bias, consent, and privacy persist.
- Health providers: Operational efficiency, resource allocation, and population health management can be enhanced, but interoperability and ROI evidence are lacking.
- Regulators and policy stakeholders: Coordinated, evidence-based policymaking is needed, with clear frameworks for liability, accountability, and post-market monitoring.
- AI developers and innovators: Standardised health data and enabling regulation are essential for scalable innovation, but SMEs face barriers in data access and compliance.
Download our snapshot below for a full summary:
Why understanding this matters
AI adoption in healthcare is not just a technical upgrade—it’s a strategic imperative for Europe’s future. The EU has laid crucial foundations through the EMA, AI Act, and European Health Data Space initiatives, but national alignment and impact modelling are needed to realise the full benefits. Understanding how the EU frames AI adoption helps stakeholders identify opportunities and risks in a global competitive environment. Europe must not only adopt innovation but actively shape the global standards that define it.
Global competitive context
As the US, China, and UK accelerate investment and integration of AI technologies, Europe must leverage its strengths—robust R&D, regulatory foresight, and commitment to ethical standards—while addressing gaps in country-level analysis, impact modelling, and best practice dissemination.
Unanswered Questions & What’s Next
The study leaves several critical questions open:
- How will the EU address gaps in country-level analysis and impact modelling?
- What lessons can be learned from underrepresented regions, especially Central and Eastern Europe?
- How can Europe ensure that AI adoption leads to measurable improvements in clinical and economic outcomes?
CF will continue to track these developments. This is the first in a series of CF snapshots on AI in European healthcare. Stay tuned!
To find the full report click here.